When human papillomavirus (HPV) was first linked to cancer in the early 1980s, it upended the way many biologists thought about viruses and cancer. By the mid-1990s, HPV was recognized globally as a key driver of cervical cancer — paving the way for the development of viral vaccines that have helped prevent this dangerous disease. Despite the success of these vaccines, HPV still contributes to nearly 4.5% of cancers today.
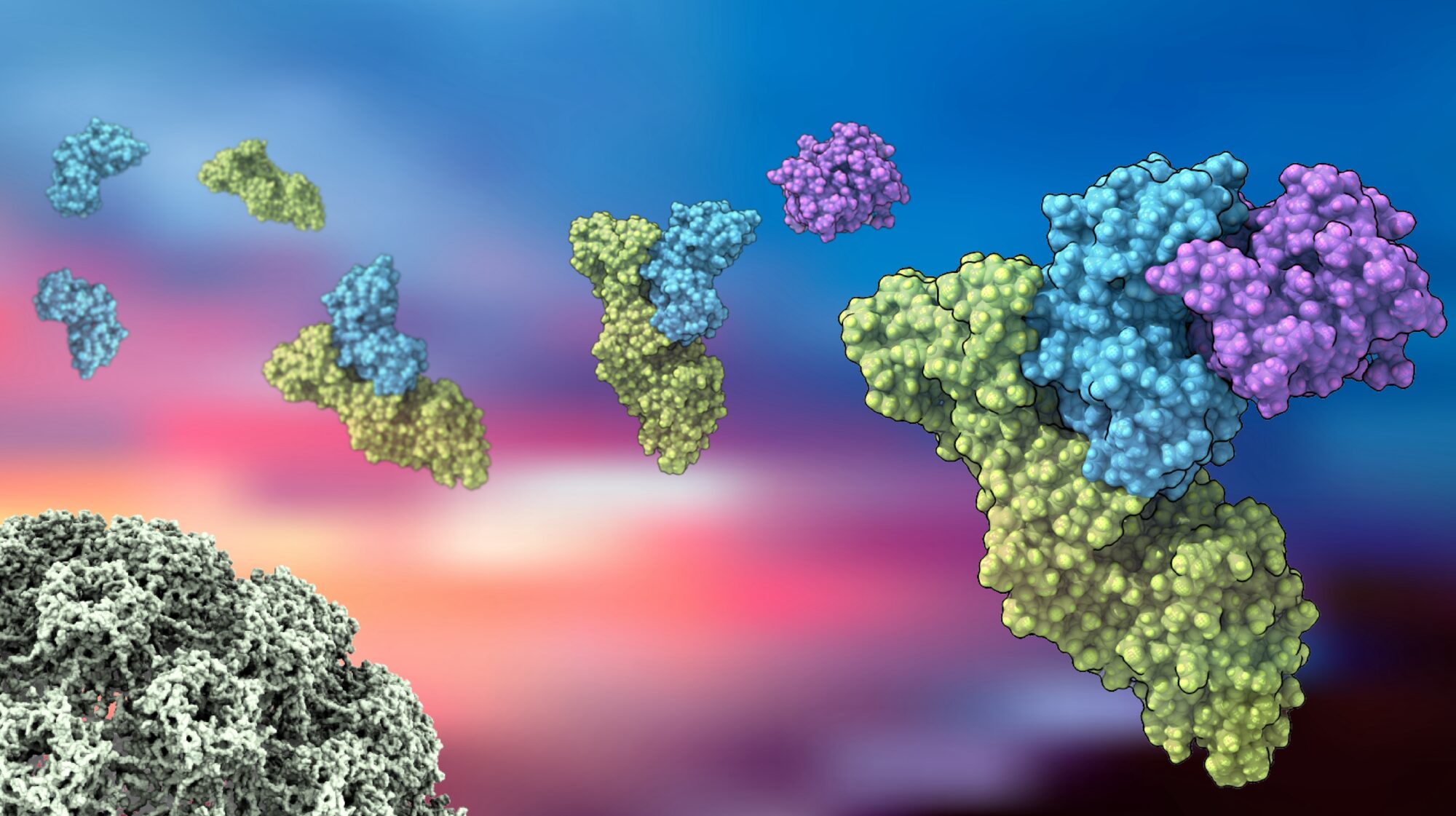
Extensive research has helped characterize more than 200 different HPV strains, but some mechanisms of HPV’s biological cascade that leads to cancer have remained a mystery. At Calico, scientists are working to make a difference by reevaluating how HPV causes disease, using a powerful structural characterization tool called cryoEM, to better understand the interaction of viral and human proteins.
In work reported in Nature Communications, Calico scientists described how high-risk, or cancer-causing, HPV proteins engage within cells, tightly binding to human proteins and altering cellular functions by degrading regulatory biomolecules. This challenges the prevailing belief that these HPV proteins have weak interactions with cellular components. Revisiting this biology has unveiled crucial discoveries with promising implications for human health and helps explain why previous attempts to develop HPV-directed therapeutics have not succeeded.
“Each finding brings us closer to our ultimate objective: identifying an effective therapeutic strategy against HPV-related cancers,” says Aaron Nile, Principal Investigator at Calico.
Expanding the Toolkit Through Collaboration
“Incorporating modern technologies, like molecular modeling in protein sciences or infusing advancements in peptide chemistry into screening platforms, is essential for developing creative and effective strategies to combat diseases like HPV and reduce their widespread impact on global health,” says John Wang, Principal Research Associate at Calico. To extend the impact of this work, Calico researchers partnered with Brad Pentelute’s lab at MIT, drawing on their expertise in developing methods to modulate protein properties and functions in order to create new tools that block HPV’s cancer-causing interactions.
Using these methods, Calico scientists engineered protein fragments called peptides capable of irreversibly inhibiting a high-risk HPV protein – the team reported on this in Chemical Science last year. Building on this success, Calico researchers developed a method to screen millions of covalent peptides, identifying specific inhibitors for a range of disease targets. The team’s latest work in JACS shows that it is possible to create selective peptide inhibitors targeting proteins associated with diverse cancers. This technique broadened our scope, introducing an innovative approach for screening covalent peptides for drug discovery and development.
“Given the importance of peptide-based therapeutics, such as semaglutide (Ozempic), developing robust and rapid screening strategies is crucial to exploit difficult-to-drug targets,” says Nile. “With these tools, we can lower the barrier for testing interesting targets and ideas, moving them more quickly into the pipeline.”