Answering fundamental questions in biology requires the ability to look closely at the intricate and microscopic world of cells and proteins. At Calico, our scientists are combining the power of Artificial Intelligence (AI) and advanced microscopy to deliver high definition structural descriptions of proteins that enable us to look deeper into the functional mechanisms of these important players in age-related disease.
One protein of interest to scientists is pregnancy-associated plasma protein A (PAPP-A), a key regulator of insulin-like growth factor 1 (IGF-1) signaling – a signaling pathway first identified by Cynthia Kenyon to slow down aging in C. elegans – and shown to influence cancer, diabetes, cardiovascular, neurodegenerative disorders and other aging related diseases (reference: 1 and 2.) With few high-resolution models of this very large protein, many fundamental questions about its functionality remain.
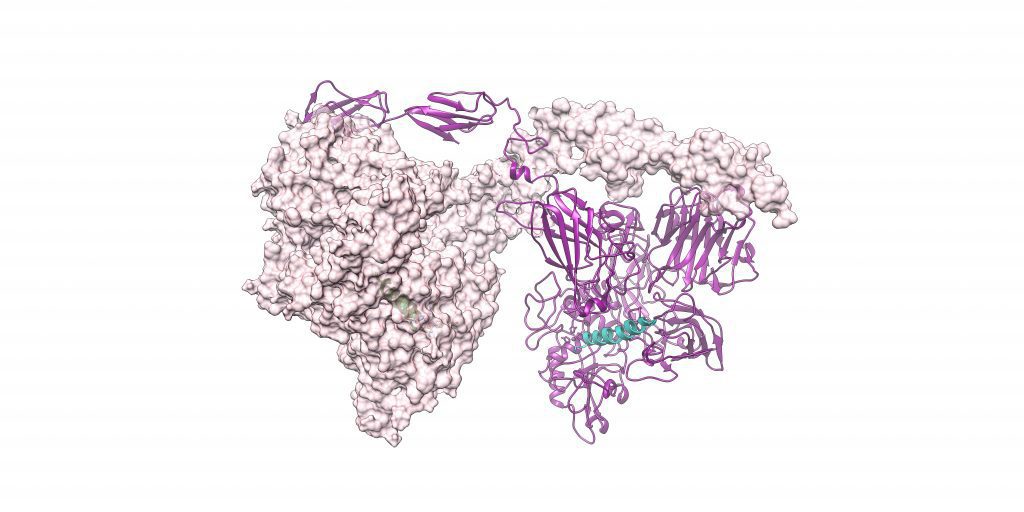
To address this knowledge gap, Calico scientists along with research partners from AbbVie and Alphabet’s DeepMind built a more complete model of PAPP-A. Their work was recently published in Nature Communications, unveiling for the first time a new high-resolution description of this protein’s native structure and its substrate complex – the structure PAPP-A adopts when bound to a small chain of amino acids, known as a peptide. As a result, these findings could inform the development of potential novel therapies for age-related diseases.
Utilizing advanced technologies to uncover PAPP-A structure
To create this new model of PAPP-A, the team developed a unique approach that utilized two very different, yet complementary technologies: single-particle cryogenic-electron microscopy (cryo-EM) and AlphaFold, an AI-powered tool built by DeepMind to construct representations of protein structures.
By combining these two approaches, the research team was able to reconstruct 3D cryo-EM structures of PAPP-A in complex with its substrate IGFBP5, and also in its native form. The structural insights, together with biochemistry studies, sheds light on the functional properties of PAPP-A and how it folds to bind and interact with IGFBP5.
“This has been a very exciting project that demonstrates the power of interdisciplinary approaches in discovery research. By combining the best aspects of cryo-EM and predictive modeling using AlphaFold, we were able to create an entirely new tool that has enabled us to take a much closer look at PAPP-A and its mechanisms for binding and selectivity in this substrate,” said co-author and Calico scientist Qi Hao.
Read the full article “Structure of the PAPP-ABP5 complex reveals mechanism of substrate recognition” in Nature Communications.